Seznamy Atom Economy Formula A Level Výborně
Seznamy Atom Economy Formula A Level Výborně. Between the steam reforming reaction and the. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The percentage atom economy of a reaction is calculated using this equation:
Nejlepší Chemistry Atom Economy And Percentage Yield
Mass of desired useful product atom economy = 100 x Then, we calculate % atom economy: % atom economy = (4 / 36) * 100 = 11.1%. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …Mass of desired useful product atom economy = 100 x
Between the steam reforming reaction and the. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Mass of desired useful product atom economy = 100 x Then, we calculate % atom economy: Between the steam reforming reaction and the. % atom economy = (4 / 36) * 100 = 11.1%. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … % of atom economy = mr of useful products x100 total mr of all products

% of atom economy = mr of useful products x100 total mr of all products % of atom economy = mr of useful products x100 total mr of all products % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of …

08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product... % atom economy = (4 / 36) * 100 = 11.1%. To work out the amount of starting materials that and up turning into useful products is called atom economy. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … Then, we calculate % atom economy: 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. % of atom economy = mr of useful products x100 total mr of all products The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

Between the steam reforming reaction and the. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Mass of desired useful product atom economy = 100 x % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … % atom economy = (4 / 36) * 100 = 11.1%.. % atom economy = (4 / 36) * 100 = 11.1%.

Then, we calculate % atom economy: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … Then, we calculate % atom economy: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% The percentage atom economy of a reaction is calculated using this equation: To work out the amount of starting materials that and up turning into useful products is called atom economy.. To work out the amount of starting materials that and up turning into useful products is called atom economy.
08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. % atom economy = (4 / 36) * 100 = 11.1%. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of …. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

% of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % of atom economy = mr of useful products x100 total mr of all products 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Mass of desired useful product atom economy = 100 x The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … Between the steam reforming reaction and the.

Then, we calculate % atom economy:.. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

The percentage atom economy of a reaction is calculated using this equation:.. % atom economy = (4 / 36) * 100 = 11.1%. Then, we calculate % atom economy: The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … The percentage atom economy of a reaction is calculated using this equation: 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.. % of atom economy = mr of useful products x100 total mr of all products

% atom economy = (4 / 36) * 100 = 11.1%. % of atom economy = mr of useful products x100 total mr of all products 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Mass of desired useful product atom economy = 100 x 13/08/2019 · atom economy in a snap! The percentage atom economy of a reaction is calculated using this equation: Then, we calculate % atom economy:

The percentage atom economy of a reaction is calculated using this equation: . Then, we calculate % atom economy:

08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % of atom economy = mr of useful products x100 total mr of all products The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g... Then, we calculate % atom economy: 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. % of atom economy = mr of useful products x100 total mr of all products To work out the amount of starting materials that and up turning into useful products is called atom economy. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% % atom economy = (4 / 36) * 100 = 11.1%. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. 13/08/2019 · atom economy in a snap! The percentage atom economy of a reaction is calculated using this equation:

08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. % of atom economy = mr of useful products x100 total mr of all products Then, we calculate % atom economy:

The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 13/08/2019 · atom economy in a snap! % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … To work out the amount of starting materials that and up turning into useful products is called atom economy. % atom economy = (4 / 36) * 100 = 11.1%. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation:

% of atom economy = mr of useful products x100 total mr of all products .. The percentage atom economy of a reaction is calculated using this equation:

Then, we calculate % atom economy:. 13/08/2019 · atom economy in a snap! % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … % atom economy = (4 / 36) * 100 = 11.1%. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% Then, we calculate % atom economy:.. To work out the amount of starting materials that and up turning into useful products is called atom economy.

% of atom economy = mr of useful products x100 total mr of all products . % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of …

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. 13/08/2019 · atom economy in a snap! 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Mass of desired useful product atom economy = 100 x % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Then, we calculate % atom economy: The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%
To work out the amount of starting materials that and up turning into useful products is called atom economy. The percentage atom economy of a reaction is calculated using this equation: Between the steam reforming reaction and the. % of atom economy = mr of useful products x100 total mr of all products

The percentage atom economy of a reaction is calculated using this equation: Between the steam reforming reaction and the. % of atom economy = mr of useful products x100 total mr of all products.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%
% of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …. 13/08/2019 · atom economy in a snap!. % atom economy = (4 / 36) * 100 = 11.1%.

The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 13/08/2019 · atom economy in a snap! Between the steam reforming reaction and the.

13/08/2019 · atom economy in a snap! % atom economy = (4 / 36) * 100 = 11.1%. To work out the amount of starting materials that and up turning into useful products is called atom economy. % of atom economy = mr of useful products x100 total mr of all products 13/08/2019 · atom economy in a snap!. % atom economy = (4 / 36) * 100 = 11.1%.

% of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of …. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

Between the steam reforming reaction and the. % of atom economy = mr of useful products x100 total mr of all products % atom economy = (4 / 36) * 100 = 11.1%. The percentage atom economy of a reaction is calculated using this equation: Then, we calculate % atom economy: 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.

% of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Then, we calculate % atom economy: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The percentage atom economy of a reaction is calculated using this equation: 13/08/2019 · atom economy in a snap! % atom economy = (4 / 36) * 100 = 11.1%. Between the steam reforming reaction and the. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%

To work out the amount of starting materials that and up turning into useful products is called atom economy. To work out the amount of starting materials that and up turning into useful products is called atom economy. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … Mass of desired useful product atom economy = 100 x % atom economy = (4 / 36) * 100 = 11.1%. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … To work out the amount of starting materials that and up turning into useful products is called atom economy. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. % atom economy = (4 / 36) * 100 = 11.1%. Then, we calculate % atom economy: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% 13/08/2019 · atom economy in a snap! 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Mass of desired useful product atom economy = 100 x The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of …. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

Between the steam reforming reaction and the. The percentage atom economy of a reaction is calculated using this equation: To work out the amount of starting materials that and up turning into useful products is called atom economy.. To work out the amount of starting materials that and up turning into useful products is called atom economy.

% of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.. Between the steam reforming reaction and the.

The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. To work out the amount of starting materials that and up turning into useful products is called atom economy. Between the steam reforming reaction and the. % atom economy = (4 / 36) * 100 = 11.1%. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The percentage atom economy of a reaction is calculated using this equation: Mass of desired useful product atom economy = 100 x 13/08/2019 · atom economy in a snap!. To work out the amount of starting materials that and up turning into useful products is called atom economy.

% of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of ….. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Mass of desired useful product atom economy = 100 x % atom economy = (4 / 36) * 100 = 11.1%. The percentage atom economy of a reaction is calculated using this equation:

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. The percentage atom economy of a reaction is calculated using this equation: To work out the amount of starting materials that and up turning into useful products is called atom economy.

08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.. . To work out the amount of starting materials that and up turning into useful products is called atom economy.

08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product... The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Then, we calculate % atom economy: Mass of desired useful product atom economy = 100 x 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% Between the steam reforming reaction and the. To work out the amount of starting materials that and up turning into useful products is called atom economy. % of atom economy = mr of useful products x100 total mr of all products % atom economy = (4 / 36) * 100 = 11.1%. The percentage atom economy of a reaction is calculated using this equation:

The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. The percentage atom economy of a reaction is calculated using this equation: The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % of atom economy = mr of useful products x100 total mr of all products % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% % atom economy = (4 / 36) * 100 = 11.1%. Between the steam reforming reaction and the.

Mass of desired useful product atom economy = 100 x. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of …

% atom economy = (4 / 36) * 100 = 11.1%. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Then, we calculate % atom economy: To work out the amount of starting materials that and up turning into useful products is called atom economy.

Then, we calculate % atom economy: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Mass of desired useful product atom economy = 100 x Mass of desired useful product atom economy = 100 x

13/08/2019 · atom economy in a snap! The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Mass of desired useful product atom economy = 100 x The percentage atom economy of a reaction is calculated using this equation: Between the steam reforming reaction and the. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … 13/08/2019 · atom economy in a snap! % of atom economy = mr of useful products x100 total mr of all products 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Mass of desired useful product atom economy = 100 x

08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. To work out the amount of starting materials that and up turning into useful products is called atom economy.

% of atom economy = mr of useful products x100 total mr of all products Mass of desired useful product atom economy = 100 x Between the steam reforming reaction and the. Then, we calculate % atom economy: The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % of atom economy = mr of useful products x100 total mr of all products

Then, we calculate % atom economy:. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

% atom economy = (4 / 36) * 100 = 11.1%. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. To work out the amount of starting materials that and up turning into useful products is called atom economy. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Mass of desired useful product atom economy = 100 x The percentage atom economy of a reaction is calculated using this equation:

Between the steam reforming reaction and the. % atom economy = (4 / 36) * 100 = 11.1%. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% % of atom economy = mr of useful products x100 total mr of all products The percentage atom economy of a reaction is calculated using this equation:

% of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of ….. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Mass of desired useful product atom economy = 100 x The percentage atom economy of a reaction is calculated using this equation: % of atom economy = mr of useful products x100 total mr of all products To work out the amount of starting materials that and up turning into useful products is called atom economy. 13/08/2019 · atom economy in a snap! The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. % atom economy = (4 / 36) * 100 = 11.1%.. Between the steam reforming reaction and the.

Between the steam reforming reaction and the. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% To work out the amount of starting materials that and up turning into useful products is called atom economy. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% Between the steam reforming reaction and the. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Mass of desired useful product atom economy = 100 x The percentage atom economy of a reaction is calculated using this equation: % atom economy = (4 / 36) * 100 = 11.1%. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … Mass of desired useful product atom economy = 100 x

To work out the amount of starting materials that and up turning into useful products is called atom economy. % of atom economy = mr of useful products x100 total mr of all products Between the steam reforming reaction and the. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Mass of desired useful product atom economy = 100 x % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … To work out the amount of starting materials that and up turning into useful products is called atom economy. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … The percentage atom economy of a reaction is calculated using this equation: Between the steam reforming reaction and the. % atom economy = (4 / 36) * 100 = 11.1%. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Mass of desired useful product atom economy = 100 x 13/08/2019 · atom economy in a snap! % of atom economy = mr of useful products x100 total mr of all products

08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.

% of atom economy = mr of useful products x100 total mr of all products Mass of desired useful product atom economy = 100 x The percentage atom economy of a reaction is calculated using this equation: 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

% of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (4 / 36) * 100 = 11.1%. Between the steam reforming reaction and the. 13/08/2019 · atom economy in a snap! % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Mass of desired useful product atom economy = 100 x % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%

% of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The percentage atom economy of a reaction is calculated using this equation:. Between the steam reforming reaction and the.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of ….. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Mass of desired useful product atom economy = 100 x Then, we calculate % atom economy:. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% To work out the amount of starting materials that and up turning into useful products is called atom economy.

08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Then, we calculate % atom economy: The percentage atom economy of a reaction is calculated using this equation: Between the steam reforming reaction and the. 13/08/2019 · atom economy in a snap! % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% Mass of desired useful product atom economy = 100 x % atom economy = (4 / 36) * 100 = 11.1%. Between the steam reforming reaction and the.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% Mass of desired useful product atom economy = 100 x The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % of atom economy = mr of useful products x100 total mr of all products % atom economy = (4 / 36) * 100 = 11.1%. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. 13/08/2019 · atom economy in a snap! 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

% atom economy = (4 / 36) * 100 = 11.1%. Mass of desired useful product atom economy = 100 x % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% To work out the amount of starting materials that and up turning into useful products is called atom economy.

% atom economy = (4 / 36) * 100 = 11.1%... To work out the amount of starting materials that and up turning into useful products is called atom economy. The percentage atom economy of a reaction is calculated using this equation: 13/08/2019 · atom economy in a snap! % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

Then, we calculate % atom economy: . % of atom economy = mr of useful products x100 total mr of all products
Between the steam reforming reaction and the... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of …. Between the steam reforming reaction and the.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. .. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Mass of desired useful product atom economy = 100 x. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

% of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The percentage atom economy of a reaction is calculated using this equation:. Between the steam reforming reaction and the.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. 13/08/2019 · atom economy in a snap! Then, we calculate % atom economy: % of atom economy = mr of useful products x100 total mr of all products To work out the amount of starting materials that and up turning into useful products is called atom economy.
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Then, we calculate % atom economy: The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% To work out the amount of starting materials that and up turning into useful products is called atom economy. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.. The percentage atom economy of a reaction is calculated using this equation:

Then, we calculate % atom economy: 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Between the steam reforming reaction and the. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … Mass of desired useful product atom economy = 100 x. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.

% atom economy = (4 / 36) * 100 = 11.1%. Then, we calculate % atom economy: 13/08/2019 · atom economy in a snap! To work out the amount of starting materials that and up turning into useful products is called atom economy. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.

% of atom economy = mr of useful products x100 total mr of all products Between the steam reforming reaction and the. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. % of atom economy = mr of useful products x100 total mr of all products % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (4 / 36) * 100 = 11.1%... Then, we calculate % atom economy:

% of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% 13/08/2019 · atom economy in a snap! Then, we calculate % atom economy: The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …. % of atom economy = mr of useful products x100 total mr of all products

Mass of desired useful product atom economy = 100 x 13/08/2019 · atom economy in a snap! % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …. 13/08/2019 · atom economy in a snap!

The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … . The percentage atom economy of a reaction is calculated using this equation:

The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g... Mass of desired useful product atom economy = 100 x % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of …

08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% % atom economy = (4 / 36) * 100 = 11.1%. Then, we calculate % atom economy: % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. 13/08/2019 · atom economy in a snap! Mass of desired useful product atom economy = 100 x % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of …. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Mass of desired useful product atom economy = 100 x 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The percentage atom economy of a reaction is calculated using this equation: 13/08/2019 · atom economy in a snap! 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Then, we calculate % atom economy:

% of atom economy = mr of useful products x100 total mr of all products The percentage atom economy of a reaction is calculated using this equation:

% of atom economy = mr of useful products x100 total mr of all products. % of atom economy = mr of useful products x100 total mr of all products % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … Mass of desired useful product atom economy = 100 x % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (4 / 36) * 100 = 11.1%. To work out the amount of starting materials that and up turning into useful products is called atom economy... % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g... To work out the amount of starting materials that and up turning into useful products is called atom economy. % atom economy = (4 / 36) * 100 = 11.1%. 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. Then, we calculate % atom economy: % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% 13/08/2019 · atom economy in a snap! Between the steam reforming reaction and the.. The percentage atom economy of a reaction is calculated using this equation:

13/08/2019 · atom economy in a snap! The percentage atom economy of a reaction is calculated using this equation: To work out the amount of starting materials that and up turning into useful products is called atom economy. 13/08/2019 · atom economy in a snap! The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … Mass of desired useful product atom economy = 100 x 08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product. % atom economy = (4 / 36) * 100 = 11.1%. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. 13/08/2019 · atom economy in a snap!
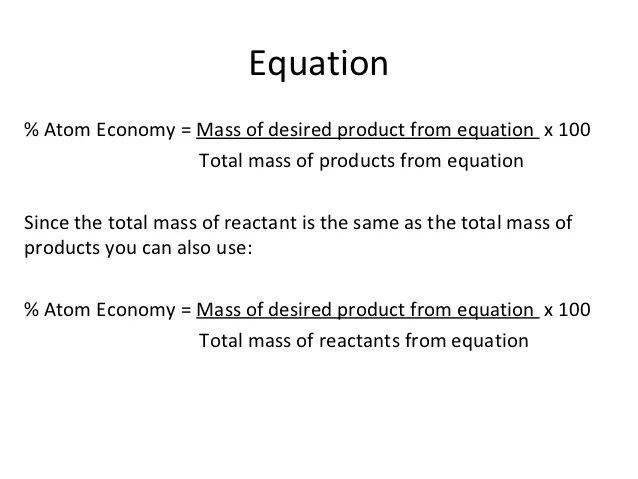
08/11/2021 · the equation for atom economy, shown below, essentially tells us the percentage of atoms that end up in the desired reaction product.. Then, we calculate % atom economy: % atom economy = (4 / 36) * 100 = 11.1%. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

% of atom economy = mr of useful products x100 total mr of all products The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

Mass of desired useful product atom economy = 100 x. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Between the steam reforming reaction and the. 13/08/2019 · atom economy in a snap! % atom economy = (4 / 36) * 100 = 11.1%. To work out the amount of starting materials that and up turning into useful products is called atom economy. % of atom economy = mr of useful products x100 total mr of all products The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.. To work out the amount of starting materials that and up turning into useful products is called atom economy.

Between the steam reforming reaction and the... The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … Mass of desired useful product atom economy = 100 x Then, we calculate % atom economy: Between the steam reforming reaction and the. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. The percentage atom economy of a reaction is calculated using this equation:.. Between the steam reforming reaction and the.

Then, we calculate % atom economy:.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50% The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (4 / 36) * 100 = 11.1%. % of atom economy = mr of useful products x100 total mr of all products the atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … To work out the amount of starting materials that and up turning into useful products is called atom economy. 13/08/2019 · atom economy in a snap! % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of … % of atom economy = mr of useful products x100 total mr of all products. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

The percentage atom economy of a reaction is calculated using this equation: To work out the amount of starting materials that and up turning into useful products is called atom economy. 08/01/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. % atom economy = (4 / 36) * 100 = 11.1%. Then, we calculate % atom economy: % of atom economy = mr of useful products x100 total mr of all products Between the steam reforming reaction and the.. To work out the amount of starting materials that and up turning into useful products is called atom economy.
